More Information
Submitted: July 03, 2024 | Approved: July 13, 2024 | Published: July 15, 2024
How to cite this article: Aromiwura A, Gandhi P, Khan M, Mattumpuram J. Post-catheterization Common Femoral Artery Pseudoaneurysm in a Patient with a Mechanical Mitral Valve Requiring Anticoagulation: A Case Report. J Radiol Oncol. 2024; 8(2): 074-00. https://dx.doi.org/10.29328/journal.jro.1001068
Copyright License: © 2024 Aromiwura A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Common femoral artery; Pseudoaneurysm; Rectus sheath hematoma; Mechanical mitral valve
Abbreviated title: ACC: American College of Cardiology; ACCP: American College of Chest Physicians; CTAP: Computed Tomography of the Abdomen and Pelvis; CFA: Common Femoral Artery; CAD: Coronary Artery Disease; CCTA: Coronary Computed Tomography Angiography; ICA: Invasive Coronary Angiography; PSA: Pseudoaneurysm; UGC: Ultrasound-Guided Compression; UGIT: Ultrasound-Guided Thrombin Injection
Post-catheterization Common Femoral Artery Pseudoaneurysm in a Patient with a Mechanical Mitral Valve Requiring Anticoagulation: A Case Report
Afolasayo Aromiwura1*, Pooja Gandhi1, Muhammad Khan2 and Jishanth Mattumpuram2*
1Department of Medicine, University of Louisville, Louisville, Kentucky, USA
2Division of Cardiology, Department of Medicine, University of Louisville, Louisville, Kentucky, USA
*Address for Correspondence: Jishanth Mattumpuram, MD, FACC, Division of Cardiology, Department of Medicine, University of Louisville, Louisville, Kentucky, USA, Email: jishanth.mattumpuram@louisville.edu
Afolasayo Aromiwura, DO, Department of Medicine, University of Louisville, Louisville, Kentucky, USA, Email: afolasayo.aromiwura@louisville.edu
Iatrogenic femoral pseudoaneurysms are a rare complication of transfemoral vascular access. We present a case of a 65-year-old woman with a mechanical mitral valve requiring warfarin, who developed a femoral pseudoaneurysm four days after cardiac catheterization with femoral access. The patient developed a 17 x 10 x 17 cm rectus sheath hematoma and was treated with ultrasound-guided thrombin injection. Anticoagulation was held for three days while the patient was monitored for further bleeding and later restarted based on shared decision-making, given the risk of valve thrombosis. There are few guidelines regarding the re-initiation of anticoagulation in high-risk bleeding patients with mechanical mitral valves. Non-invasive coronary computed tomography angiography should be considered in patients on anticoagulation who require non-emergent cardiac ischemic evaluation.
Femoral artery Pseudoaneurysm (PSA) is the most common post-catheterization complication seen after transfemoral vascular access. The incidence of post-procedure PSA ranges from 0.02 to 9%; however, a value of 0.2% was proposed by the Society of Cardiovascular and Interventional Radiology as an acceptable rate of occurrence for iatrogenic PSAs [1,2]. PSAs develop because of blood extravasation beyond the arterial wall layers into the surrounding tissue, forming a localized hematoma. The wall of the PSA is composed of a fibrous pseudo-capsule that develops and limits further extravasation. Risk factors such as anticoagulation, puncture method, arterial calcification, sheath size ≥ 7F, superior femoral or deep femoral artery puncture, hypertension, and insufficient compression after catheter removal contribute to post-catheterization PSAs [3]. A femoral PSA may be identified on physical exam by a palpable thrill, bruit, lower extremity edema, and palpable mass. The physical burden of the PSA and hematoma on nearby neurovascular structures can lead to complications, including pain, skin ischemia and necrosis, neuropathy, distal embolization, and aneurysm rupture [1].
In this scenario, we explore a case that involved the development of an iatrogenic femoral artery pseudoaneurysm and rectus sheath hematoma after cardiac catheterization in a patient with a mechanical mitral valve requiring anticoagulation with warfarin. Additionally, this report discusses challenges in managing PSA when complicated by the presence of a mechanical mitral valve requiring continued anticoagulation.
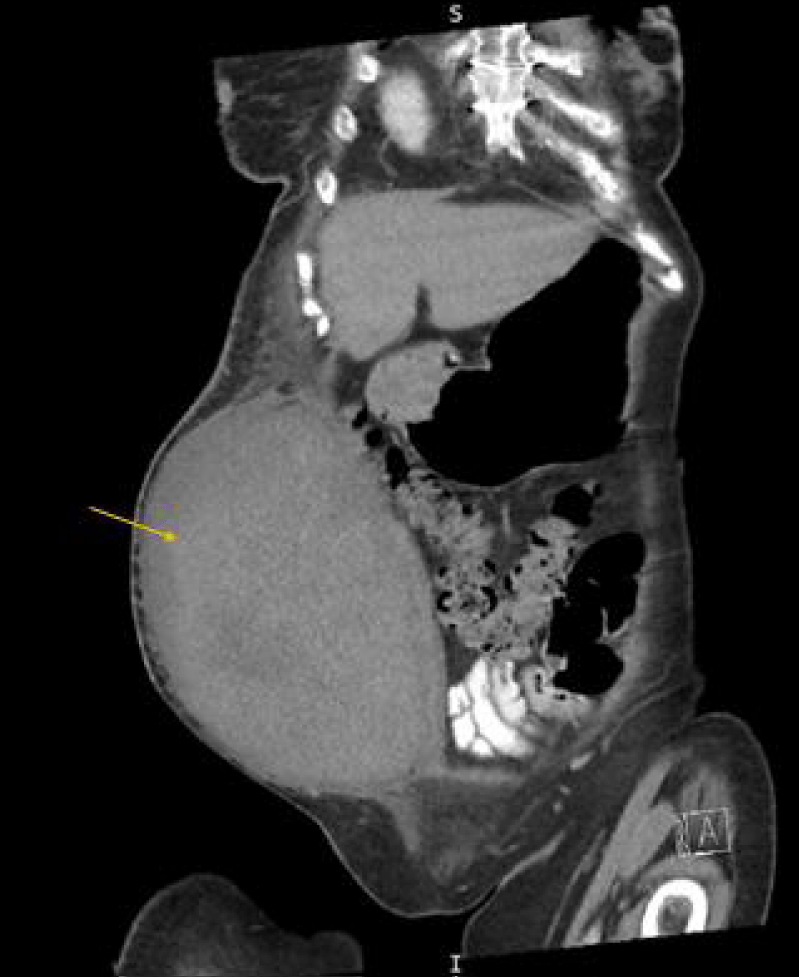
A 65-year-old woman with a history of coronary artery bypass grafting for severe coronary artery disease, mechanical mitral valve replacement on warfarin, persistent atrial fibrillation, and heart failure with reduced ejection fraction, presented with a two-day history of increased urination, fever, and bilateral flank pain. She was admitted for urosepsis and treated with intravenous fluids, vancomycin, and cefepime. Vancomycin was discontinued after blood cultures grew no bacteria at 48 hours and cefepime was continued for a total 5-day course. On day two of admission, her high sensitivity troponin increased from 24 ng/L to 1509 ng/L with EKG changes significant for atrial fibrillation with rapid ventricular response with a previously known left bundle branch block. There were no ST elevations or T wave inversions. Given her history of coronary artery disease and high suspicion of acute coronary syndrome, warfarin was transitioned to heparin, and she underwent cardiac catheterization via a right femoral access under ultrasound guidance. The Heparin drip was discontinued immediately before patient transportation for the catheterization. Right radial access was initially attempted but was unsuccessful due to radial artery vasospasm. Angiography showed no obstructive Coronary Artery Disease (CAD) and normal right heart pressure. Post-catheterization, the femoral access site was dressed with gauze and Tegaderm. Given the lack of CAD, troponin elevation was attributed to demand ischemia in the setting of sepsis. Four days post-catheterization, she developed new right lower quadrant pain and hypotension. Later the same day, the on-call team was notified that the hematoma in the right lower quadrant noted on ultrasound was rapidly expanding. Upon confirmation, the heparin was immediately discontinued, and the patient was upgraded to the medical intensive care unit. Initial computed tomography of the abdomen and pelvis (CTAP) was significant for a large right rectus sheath hematoma measuring 17 x 10 x 17 cm with concern for active extravasation from the right inferior epigastric artery. Subsequently, a right iliofemoral angiogram was performed for further characterization of the bleeding source and demonstrated a right Common Femoral Artery (CFA) hemorrhage into a PSA, not an inferior epigastric artery hemorrhage (Figure 1). The patient received Ultrasound-Guided Thrombin Injection (UGTI) with 300 units of thrombin injected into the right CFA PSA sac and closer to the neck with successful thrombosis and no further color flow within the PSA on repeat imaging. Heparin was re-started on day three, post-hematoma onset, after an in-depth risk-benefit discussion with the family. Bridge to warfarin was started on day ten post-hematoma onset after ensuring hematoma stabilization, hemoglobin stability, and no signs of skin ischemia or necrosis. 19 days after initial hematoma onset, a CTAP demonstrated the rectus sheath hematoma measuring 11.4 x 18.5 x 22.2 cm (Figure 2). The patient was discharged to an acute inpatient rehab facility and was later discharged from rehab after 14 days. Two months after the initial hematoma onset, an outpatient monitoring ultrasound was performed and showed a well-circumscribed heterogeneously hypoechoic structure measuring 15.5 x 14.0 x 10.5 cm. The patient had no further occurrences of bleeding
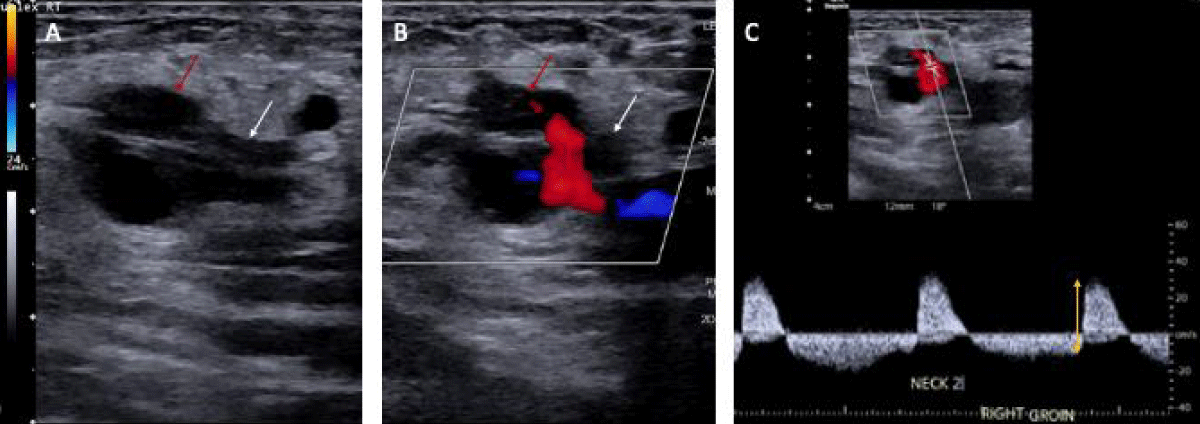
Figure 1: Two-dimensional ultrasound representation of the femoral artery pseudoaneurysm in our 65-year-old patient. A) PSA sac (red arrow) and neck (white arrow) without color flow. B) PSA sac and neck with color flow showing inflow at the neck. C) Waveform showing bidirectional flow in the PSA (yellow double-sided arrow).
Figure 2: Computed tomography of the abdomen and pelvis with contrast showing coronal view of the rectus sheath hematoma measuring approximately 11.4 x 18.5 x 22.2 cm 19 days post-onset.
Arterial duplex ultrasound is the first-line imaging modality for assessing arterial blood flow within the PSA using color Doppler [4]. Color flow within the pseudocapsule demonstrates a bidirectional blood flow pattern known as a ‘yin-yang sign’ characteristic of PSAs and larger aneurysms. Iatrogenic PSA typically develops within 24 hours post-procedure; however, it may still occur up to 7 to 10 days after the inciting event. Regardless, they should be treated emergently, especially when presenting with hemodynamic instability and a rapidly evolving hematoma, as was described in this patient.
Various approaches are utilized for femoral PSA management, including ultrasound-guided compression (UGC), UGTI, open repair, or endovascular repair. Open repair is the traditional treatment approach, but other less invasive and cost-effective methods are now favored. Open repair is employed in skin ischemia or infection, as escalation when less invasive approaches fail or are contraindicated, and in patients who are already scheduled to receive general anesthesia for other procedures [5]. UGC involves the cyclic application of a minimum amount of pressure to the PSA over 10 to 20 minutes with subsequent re-assessment of PSA flow via Doppler [5]. The cyclic compression is cautiously applied to prevent femoral artery occlusion and continues until PSA thrombosis is achieved. UGTI includes the percutaneous injection of thrombin into the pseudocapsule and may include the placement of a balloon at the pseudocapsule neck to prevent thrombin leakage into the femoral artery. UGTI is successful in 93% - 100% of cases and is superior to UGC in patients on anticoagulation and time to onset of action.
Anticoagulation with a vitamin K antagonist to an international normalized ratio of 3.0 is a class 1 recommendation by the American College of Cardiology (ACC) for patients with mechanical mitral valves [6]. The main factors to consider are the risk of thromboembolism, PSA bleeding, and further hematoma spread. According to the 2012 American College of Chest Physicians (ACCP) perioperative anticoagulation guideline, patients with mitral valve prosthesis are considered high-VTE risk [7].
A 2020 ACC expert consensus guides the re-initiation of anticoagulation depending on the location of the bleed [8]. Specialist discussion of rebleeding and thrombosis risk is suggested for patients with critical site bleeds who are willing to restart anticoagulation and have no further invasive procedures planned. Critical site bleeds include intracranial or central nervous system hemorrhages, pericardial tamponades, hemothorax, intra-abdominal bleeds, and intramuscular bleeds [8]. Intramuscular bleeds carry the risk of compartment syndrome, neuropathy, and ischemia. In this case, we employed a multi-disciplinary discussion including vascular surgery, cardiology, the primary medicine team, and the patient. The multi-disciplinary team recommended re-initiation of anticoagulation given hemoglobin and hematoma stabilization, the lack of soft tissue ischemic and compression signs such as progressive pallor and paresthesia, and patient agreement with the proposed management plan. The patient’s anticoagulation was re-initiated with heparin and she was monitored further for ischemia and rebleeding before transitioning back to warfarin.
The decision for anticoagulation management in patients with PSA and mechanical valves can be made on a case-by-case basis after specialist assessment of the risks of rebleeding and valve thrombosis, hematoma stabilization, and patient comfort.
The DISCHARGE trial compared CT and invasive coronary angiography (ICA) for guiding treatment of patients with stable chest pain and illustrated less procedure-related complications in the CT arm (0.5%) relative to the ICA arm (1.9%) (hazard ratio, 0.26%; 95% CI, 0.13 – 0.55) [9]. Given that this patient required anticoagulation, particularly warfarin, and PSA was a possible complication, other non-invasive alternatives to catheterization, such as coronary CT angiography (CCTA), could be considered for non-emergent evaluation.
Femoral artery pseudoaneurysms are a potential complication of invasive angiograms. This case describes the complexity of anticoagulation management in patients with a high risk of bleeding and valve thrombosis. Expert consensus suggests specialist-guided decision-making for anticoagulation re-initiation in patients with high rebleeding and thrombosis risk who are willing to restart anticoagulation and have no further invasive interventions planned, therefore, we utilized this approach. Nevertheless, non-invasive techniques such as CCTA may be considered in individuals with high thrombosis and bleeding risk, given the lower risk of complications with CT relative to invasive angiography.
Contributors
AA and PJ drafted and researched the manuscript. JM sourced the clinical images. AA edited the images and figures. AA, PJ, JM, and MK edited the manuscript. JM gave final approval of the manuscript.
Ethical considerations
Informed consent was obtained from the patient for publication.
- Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of post catheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-71. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0167527306013155
- Spies JB, Bakal CW, Burke DR, Burke DR, Cardella JF, Dawson SL, et al. Standard for Diagnostic Arteriography in Adults. J Vasc Interv Radiol. 1993;4(3):385-95. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1051044393718850
- Morgan R, Belli AM. Current Treatment Methods for Postcatheterization Pseudoaneurysms. J Vasc Interv Radiol. 2003;14(6):697-710. Available from: https://pubmed.ncbi.nlm.nih.gov/12817037/
- Chun EJ. Ultrasonographic evaluation of complications related to transfemoral arterial procedures. Ultrasonography. 2018;37(2):164-73. Available from: https://pubmed.ncbi.nlm.nih.gov/29145350/
- Corriere MA, Guzman RJ. True and False Aneurysms of the Femoral Artery. Semin Vasc Surg. 2005;18(4):216-23. Available from: https://pubmed.ncbi.nlm.nih.gov/16360579/
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5). Available from: https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000923
- Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative Management of Antithrombotic Therapy. Chest. 2012;141(2 Suppl). Available from: https://pubmed.ncbi.nlm.nih.gov/22315266/
- Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants. J Am Coll Cardiol. 2020;76(5):594-622. Available from: https://pubmed.ncbi.nlm.nih.gov/32680646/
- The DISCHARGE Trial Group. CT or Invasive Coronary Angiography in Stable Chest Pain. N Engl J Med. 2022;386(17):1591-602. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa2200963