More Information
Submitted: May 22, 2024 | Approved: June 03, 2024 | Published: June 04, 2024
How to cite this article: Sakhi H, Chevance V, Kalifa L, Arana R, Laparra A, et al. Fatal Immune Checkpoint Inhibitor-associated Myocarditis Mimicking Infiltrative Cardiomyopathy in a 54-year-old Woman with Metastatic Melanoma. J Radiol Oncol. 2024; 8: 046-050.
DOI: 10.29328/journal.jro.1001063
Copyright License: © 2024 Sakhi H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Myocarditis; Cardio-oncology; Infiltrative cardiomyopathy; Cardiac magnetic resonance; Immune checkpoint inhibitors
Fatal Immune Checkpoint Inhibitor-associated Myocarditis Mimicking Infiltrative Cardiomyopathy in a 54-year-old Woman with Metastatic Melanoma
Hichem Sakhi1* , Virgile Chevance1, Laurette Kalifa1, Riad Arana2, Ariane Laparra3, Guillaume Reverdito1, Fares Ben Salem1, Charles Pottier4, Olivier Lambotte5, Arshid Azarine1*# and Sondes Smaali1,6,7#
, Virgile Chevance1, Laurette Kalifa1, Riad Arana2, Ariane Laparra3, Guillaume Reverdito1, Fares Ben Salem1, Charles Pottier4, Olivier Lambotte5, Arshid Azarine1*# and Sondes Smaali1,6,7#
1Cardiovascular Imaging Unit, Department of Medical Imaging, Hôpital Marie Lannelongue, Fondation Paris Saint Joseph, Paris-Saclay University, Le Plessis-Robinson, France
2Department of Pathology, Hôpital Marie Lannelongue, Fondation Paris Saint Joseph, Paris-Saclay University, Le Plessis-Robinson, France
3Department of therapeutic Innovation and Early Trials, Gustave Roussy Institute, Paris-Saclay University, Villejuif, France
4Department of Medical Oncology, Gustave Roussy Institute, Paris-Saclay University, Villejuif, France
5Department of Internal Medicine, Hôpital Kremlin-Bicêtre, Paris-Saclay University, Le Kremlin Bicêtre, France
6Department of Cardiology, Hôpital Marie Lannelongue, Fondation Paris Saint Joseph, Paris-Saclay University, Le Plessis-Robinson, France
7Cardio-Oncology Unit, Gustave Roussy Institute, Paris-Saclay University, Villejuif, France
#Contributed equally and share the last authorship position
*Address for Correspondence: Dr. Arshid Azarine, Cardiovascular Imaging Unit, Department of Medical Imaging, Marie Lannelongue Hospital, Paris Saint-Joseph Foundation, 133 Av. de la Résistance, 92350 Le Plessis-Robinson, France, Email: aazarine@ghpsj.fr
Dr. Hichem Sakhi, Cardiovascular Imaging Unit, Department of Medical Imaging, Marie Lannelongue Hospital, Paris Saint-Joseph Foundation, 133 Av. de la Résistance, 92350 Le Plessis-Robinson, France, E-mail: hichem.sakhi@gmail.com
Introduction: Immune checkpoint inhibitors (ICI) have significantly improved cancer treatment outcomes, but cardiovascular complications such as ICI-associated myocarditis are a major concern. Diagnosing myocarditis requires integrating biomarkers, electrocardiogram (EKG), cardiac imaging, and endomyocardial biopsy. We present a case illustrating these diagnostic challenges, involving a female patient treated with pembrolizumab who developed fatal acute myocarditis mimicking infiltrative cardiomyopathy.
Case report: A 54-year-old woman with mucosal melanoma, treated with pembrolizumab, was hospitalized in May 2023 due to dyspnea and elevated troponin levels. Initial cardiac workups were normal, but subsequent tests revealed borderline cardiac magnetic resonance imaging findings. In late May 2023, the patient was admitted with worsening dyspnea, elevated NT-pro-BNP, and severe hyperlactatemia. Imaging and endomyocardial biopsy confirmed acute myocarditis with atypical presentation, mimicking infiltrative cardiomyopathy. Despite aggressive immunosuppressive therapy, the patient’s condition deteriorated, resulting in cardiogenic shock and death seven days post-admission.
Conclusion: This case underscores the diagnostic and management challenges of ICI-associated myocarditis, particularly with atypical presentations. It highlights the need for vigilant, comprehensive monitoring and further research to improve diagnostic and therapeutic strategies for managing these severe side effects in patients undergoing ICI therapy.
Immune checkpoint inhibitors (ICI) have emerged as a promising treatment in oncology, significantly improving the prognosis of patients undergoing cancer treatment [1]. ICI functions by blocking proteins that inhibit the immune system’s ability to attack cancer cells. Specifically, ICI targets checkpoint proteins such as PD-1, PD-L1, and CTLA-4, which are often exploited by tumors to evade immune detection. By inhibiting these checkpoints, ICI enhances the immune response against cancer cells [2]. Cardiovascular complications, in particular ICI-associated myocarditis, are a major concern. The cardiotoxic effects of ICI are likely due to an excessive immune response that causes inflammation and damage to all cardiac tissues. Various cardiac conditions have been associated with ICI. These include myocarditis (incidence ranging from 0.06% to 2.4%), pericardial effusion (2%), pericarditis (1%), cardiac arrhythmias (4%), atherosclerotic events (< 1%), heart failure (0.4%), and some reported cases of Tako-Tsubo syndrome [1,3]. Its diagnosis is not always simple, and it is currently recommended to integrate a combination of biomarkers, electrocardiogram (EKG), cardiac imaging, and endomyocardial biopsy to confirm potential ICI-associated myocarditis [4]. We aim to illustrate this diagnostic challenge through a case featuring a rare presentation of ICI-associated myocarditis. Here, in this clinical case challenge, we describe a female patient who received pembrolizumab treatment and developed fatal acute ICI-associated myocarditis, presenting symptoms mimicking infiltrative cardiomyopathy.
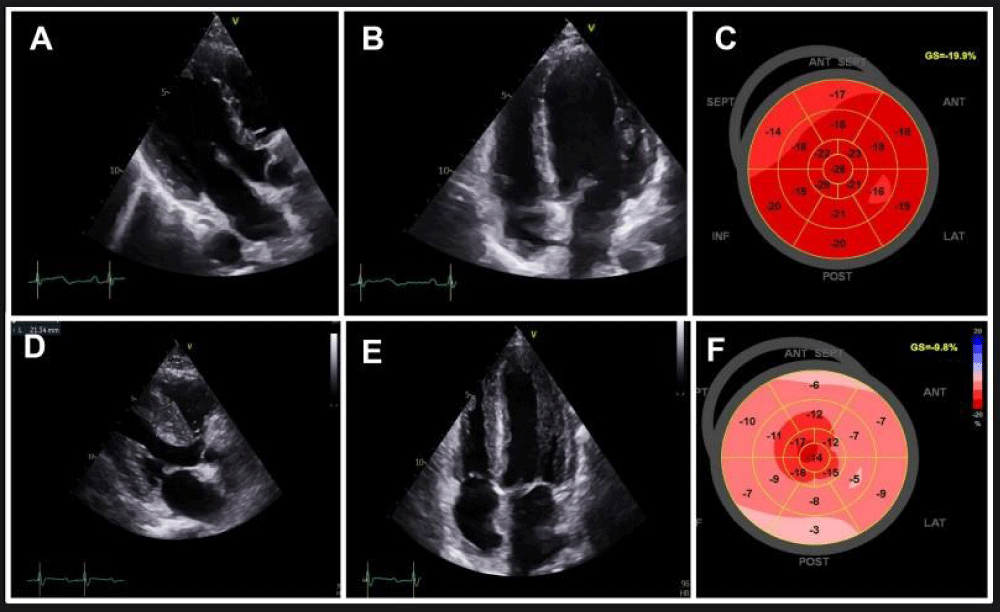
Figure 1: Trans-thoracic echocardiography findings in March 2023 (A, B, C) and in May 2023 (D, E, F).
A-B: Parasternal long-axis and four-chamber echocardiographic view demonstrating normal left ventricular wall thickness.
C: Global longitudinal strain normal at -19.9%.
D-E: Parasternal long-axis and four-chamber echocardiographic view demonstrating increased left ventricular wall thickness.
F: Global longitudinal strain decreased at -9.8%
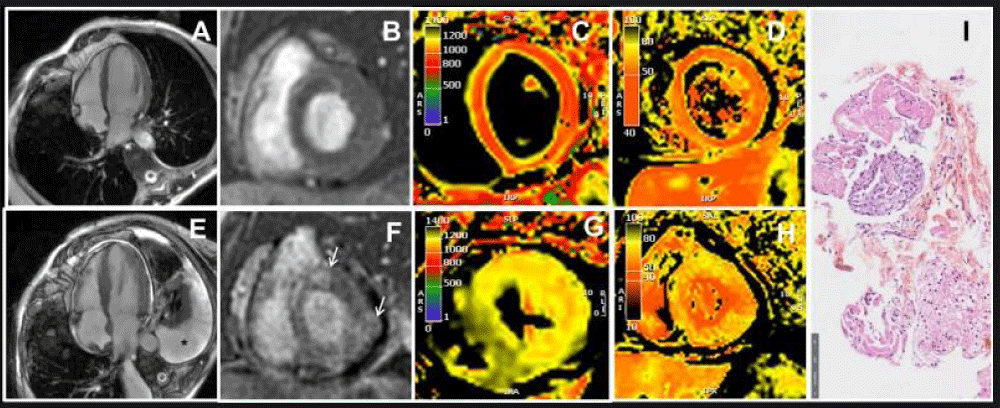
Figure 2: CMR and histologic findings in March 2023 (A, B, C, D) and in May 2023 (E, F, G, H, I).
A: Four-chamber view on cine-steady state free precession CMR sequence showing normal left ventricular wall thickness.
B: Late gadolinium enhancement sequence showing no abnormalities.
C: T1 mapping sequence indicating an absence of T1 elevation.
D: T2 mapping sequence indicating an absence of T2 elevation.
E: Four-chamber view on cine-steady state free precession CMR sequence showing increased left ventricular wall thickness. Pleural effusion can be seen (black star).
F: Late gadolinium enhancement sequence showing late enhancement in the lateral and antero-septal wall (white arrows).
G: T1 mapping sequence revealing an increase in T1.
H: T2 mapping sequence revealing an increase in T2.
I: Upon staining with hematoxylin-eosin, histological images of the endomyocardial biopsy reveal myocardial tissue containing inflammatory cells (neutrophils and mononuclear cells) and areas of myocyte damage.
A 54-year-old woman with a history of mucosal melanoma since 2022 was hospitalized in May 2023 due to shortness of breath and elevated troponin levels. Because of a recurrence of metastatic melanoma, she participated in an immunotherapy trial focused on lymphocyte targeting (specifically γδ T-cell-based cancer immunotherapy) and pembrolizumab from December 2022 to February 2023. Subsequently, she received treatment with pembrolizumab alone.
In December 2022, an initial cardiac workup, including EKG and transthoracic echocardiography (TTE), showed no structural heart disease (LVEF 66%, mean global longitudinal strain (GLS) -19.9%) (Figure 1 A-C). Cardiac biomarkers (troponin and NT-pro-BNP) were within normal limits. By March 2023, during a routine medical follow-up, the patient, who remained asymptomatic, had troponin levels twice the normal range without concurrent elevation in CK. Liver function tests and TSH were within normal range. Follow-up EKG and TTE remained unremarkable.
The coronary computed tomography scan did not reveal any abnormalities. The cardiac magnetic resonance imaging (CMR) showed borderline T2 values around 57 ms on the septum, but there were no abnormalities in T1 and extracellular volume. There was no late gadolinium enhancement. The diagnosis of myocarditis was not retained according to the modified Lake Louise criteria (Figure 2 A-D). Control troponin level remained at 2 times normal, and the patient was still asymptomatic. A CMR check-up at 3 months was initially scheduled. Pembrolizumab was resumed after all explorations.
In late May 2023, the patient was admitted to the cardiac intensive care unit due to quickly worsening dyspnea. Her NT-pro-BNP level had significantly increased to 4300 pg/mL, and her troponin level was three times the normal limit. She exhibited severe hyperlactatemia at 6 mmol/L. This hyperlactatemia remained stable, plateauing around 5-6 mmol/L, despite the initial absence of other signs of peripheral hypoperfusion for several days. On EKG, first-degree atrioventricular block and incomplete right bundle branch block were observed. TTE revealed left ventricular hypertrophy with a hyper-echogenic myocardium. While the left ventricular ejection fraction was normal (62%), the global longitudinal strain was altered with apical sparing (GLS = -9.8%). The right ventricle wall was also thickened and there was a pericardial effusion (Figure 1 D-F). CMR was performed and revealed features of acute myocarditis with diffuse myocardial edema and the presence of late gadolinium enhancement predominantly in the lateral wall, forming a very nodular shape (Figure 2 E-H). There was also a widespread and significant increase in extracellular volume between 45% and 50%. A pericardial effusion and right ventricle hypertrophy were also present. Left ventricular ejection fraction was preserved (69%). An abdominopelvic computed tomography scan performed due to hyperlactatemia revealed a heterogeneously enlarged liver and spleen with multiple secondary lesions, extensive intraperitoneal effusion, and mesenteric infiltration which could explain the hyperlactatemia.
An endomyocardial biopsy confirmed ICI-associated acute myocarditis. On hematoxylin-eosin stain, histologic images from endomyocardial biopsy show myocardial tissue with inflammatory cells (neutrophils and mononuclear cells) and foci of myocyte damage (Figure 2 I).
Treatment included corticosteroids, mycophenolate mofetil (CellCept), and intravenous immunoglobulin. Due to the serious nature of myocarditis (reduced cardiac output, elevated lactate levels, conduction disturbances), we immediately combined methylprednisone with an immunosuppressive treatment (mycophenolate mofetil). When we observed worsening cardiac output decline, ongoing troponin elevation, and conduction disturbances, we introduced intravenous immunoglobulins. Unfortunately, the patient’s condition still deteriorated with the development of a complete atrioventricular block necessitating a temporary pacemaker. Later, the patient developed cardiogenic shock, reminiscent of presentations observed in restrictive cardiomyopathies such as amyloidosis. While the left ventricular ejection fraction was still preserved, there was a severe diastolic dysfunction with a restrictive mitral inflow pattern, an altered longitudinal strain, and a low cardiac output that did not respond to treatment with noradrenaline and dobutamine. The patient died seven days after admission.
This case emphasizes the potential for serious cardiovascular complications, including fatal acute myocarditis, in patients undergoing ICI treatment and the initial diagnostic challenges. Indeed, as early as March, as part of systematic monitoring, the patient demonstrated a twofold increase in the normal troponin level and the T2 value was borderline. Nevertheless, the T1, extracellular volume, and late gadolinium enhancement were strictly normal, and the diagnosis of myocarditis was not retained according to European guidelines [4,5]. CMR was potentially suggestive but there were no other minor criteria associated. After a collegial discussion and considering the prognosis of her melanoma, it was decided to continue ICI treatment. CMR with the updated 2018 Lake Louise Criteria is the preferred non-invasive technique for diagnosing myocarditis, but its performances are challenged by ICI-associated myocarditis. The updated Lake Louise criteria include late gadolinium enhancement, and T1 and T2 mapping, which enhance the detection of edema, hyperemia, fibrosis, and necrosis [5,6]. Extracellular volume, typically around 30% in patients with myocarditis, can be a useful addition to this diagnostic toolkit, though its sensitivity (45%) and specificity (84%) are moderate. ECV values rarely exceed 40% in myocarditis patients, with values above 40% being more indicative of infiltrative cardiomyopathy, such as amyloidosis. In our patient, LV wall thickness, LGE, T2, T1, and ECV all increased significantly within one week, indicating a rapid and widespread progression of myocardial inflammation and edema. This extensive inflammation may have led to an increase in the extracellular matrix, resulting in an exceptional ECV increase above 40%, mimicking an amyloidosis-like condition. Interestingly, without the initial CMR, subsequent imaging could have misinterpreted the findings as amyloidosis-like hypertrophic LV with elevated T2. Our case highlights also the importance of performing two CMR scans within a short period in these patients: the first might have missed the disease, while the second alone could have potentially misled the diagnosis toward cardiac amyloidosis. Therefore, given the diagnostic challenge of these forms of ICI-associated myocarditis, it is essential to consider all parameters, especially their evolution: clinical presentation, EKG, troponin levels, TTE, and thus CMR. Endomyocardial biopsy remains the gold standard but unfortunately can lead to false negatives and remains an invasive procedure. Therefore, our case underscores the challenges in the initial stages of diagnosis ICI-associated myocarditis. This diagnostic issue is crucial as it determines whether to halt or continue treatment. Incorrect continuation can lead to fatal cardiac complications, while incorrect cessation can deprive a patient of essential cancer therapy.
Another very interesting element of this case is its atypical initial presentation. Hypertrophic presentations are very rarely described in the literature [7-10]. However, it’s important to note that no cases of ICI-associated myocarditis have been reported in this form previously. These cases of viral myocarditis have exhibited hypertrophy linked to substantial inflammation. Most of these cases reported had a favorable outcome with regression of hypertrophy. Myocarditis with eosinophils and giant cells can rarely present with hypertrophy [11,12]. In our case, endomyocardial biopsy ruled out this type of myocarditis.
Beyond hypertrophy, some features here could suggest amyloidosis (the diffuse increase in extracellular volume, right ventricular hypertrophy, pericardial effusion, restrictive mitral inflow). In our case, we have excluded the diagnosis of amyloidosis with normal bone scan and protein electrophoresis. The evolution of different imaging modalities and the endomyocardial biopsy strongly suggests a case of ICI-associated myocarditis. The examination revealed necrosis with nuclear neutrophil and macrophage presence in the biopsy, occurring within the context of hypertrophy. However, another unusual aspect was the lack of lymphocytes. There was no evidence of hypereosinophilia. Viral serology as well as blood and myocardium Next Generation Sequencing did not indicate any signs of infection. In our case, ICI has probably led to infiltrative cardiomyopathy by causing a rapid accumulation of neutrophils and macrophages in the cardiac muscle. This intense immune response resulted in significant inflammation and cellular infiltration, which can alter the heart’s structure and function. Specifically, the influx of these immune cells increased the thickness of the myocardial walls, leading to hypertrophy. This structural change is accompanied by physiological alterations, particularly impaired left ventricular filling, which disrupt normal cardiac function. The resultant diastolic dysfunction is characterized by restrictive filling patterns, highlighting the severity of the immune-mediated damage induced by this therapy.
This very atypical presentation deserves reporting and appears to have a poor prognosis in our case, with unfavorable evolution despite several lines of immunosuppressive treatment. Our treatment choices were guided by our experience and the ESC guidelines. In fact, while corticosteroid therapy is well-established as the first-line treatment, the choice of second-line treatments is less defined and often depends on the practices of the specific center [4].
Finally, the plateau hyperlactatemia without initial signs of low cardiac output or other signs of peripheral hypoperfusion is also an atypical feature of this case. The suggested cause was mesenteric infiltration, and it could be an effect of immunotherapy, although this cannot be confirmed and is poorly described in the literature [13]. The differential diagnosis would be muscular involvement with mitochondrial cytopathy which cannot be eliminated.
This case illustrates the complexity and challenges faced in the diagnosis and management of ICI-associated myocarditis, particularly when presenting with an atypical infiltrative pattern. It underscores the need for heightened vigilance and comprehensive multi-modal investigation in patients undergoing ICI treatment, even when initial symptoms may be nonspecific. It further highlights the need for research to improve our understanding of such cases and to develop optimal therapeutic strategies to manage these serious side effects of a highly beneficial cancer treatment. This patient’s unfortunate outcome stresses the importance of these efforts.
Ethical consideration
Informed consent was obtained from the patient’s family for the case details and its publication.
- Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018 Sep;19(9):e447-e458. doi: 10.1016/S1470-2045(18)30457-1. PMID: 30191849.
- Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, Ladwa R, O'Byrne K, Kulasinghe A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022 Apr 24;29(5):3044-3060. doi: 10.3390/curroncol29050247. PMID: 35621637; PMCID: PMC9139602.
- Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018 Dec;19(12):1579-1589. doi: 10.1016/S1470-2045(18)30608-9. Epub 2018 Nov 12. PMID: 30442497; PMCID: PMC6287923.
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022 Nov 1;43(41):4229-4361. doi: 10.1093/eurheartj/ehac244. Erratum in: Eur Heart J. 2023 May 7;44(18):1621. PMID: 36017568.
- Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018 Dec 18;72(24):3158-3176. doi: 10.1016/j.jacc.2018.09.072. PMID: 30545455.
- Chabior A, Tymińska A, Pawlak A, Giordani A, Caforio A, Grabowski M, Ozierański K. Advances in myocarditis management in the light of the latest research and recent guidelines of the European Society of Cardiology. Cardiol J. 2024;31(2):342-351. doi: 10.5603/cj.95175. Epub 2024 Jan 22. PMID: 38247433; PMCID: PMC11076022.
- Sharrack N, Poenar AM, Simms AD, Greenwood JP, Plein S. Acute Myocarditis Mimicking Hypertrophic Cardiomyopathy in Marfan Syndrome and Morphologically Abnormal Mitral Valve. JACC Case Rep. 2022 Jan 19;4(2):105-110. doi: 10.1016/j.jaccas.2021.11.023. PMID: 35106495; PMCID: PMC8784724.
- Hiramitsu S, Morimoto S, Kato S, Uemura A, Ohtsuki M, Kato Y, Sugiura A, Miyagishima K, Mori N, Yoda R, Mori K, Iwase M, Hishida H. Significance of transient left ventricular wall thickening in acute lymphocytic myocarditis. Heart Vessels. 2007 Jan;22(1):25-9. doi: 10.1007/s00380-006-0933-1. Epub 2007 Jan 26. PMID: 17285442.
- Hauser AM, Gordon S, Cieszkowski J, Timmis GC. Severe transient left ventricular "hypertrophy" occurring during acute myocarditis. Chest. 1983 Feb;83(2):275-7. doi: 10.1378/chest.83.2.275. PMID: 6217952.
- Dimarco A, Mansoubi H, Tanner M. An unusual cause of left ventricular hypertrophy. Eur Heart J. 2016 Feb 1;37(5):497. doi: 10.1093/eurheartj/ehv530. Epub 2015 Oct 22. PMID: 26494864.
- Coffin ST, Benton SM Jr, Lenihan DJ, Naftilan AJ, Mendes LA. Eosinophilic myocarditis-an unusual cause of left ventricular hypertrophy. Am J Med Sci. 2015 Apr;349(4):358-62. doi: 10.1097/MAJ.0000000000000344. PMID: 25325192.
- Liu S, Zheng L, Shen L, Wu L, Yao Y. Clinical Identification and Characteristic Analysis of Giant Cell Myocarditis in 12 Cases. Front Cardiovasc Med. 2021 Apr 13;8:649094. doi: 10.3389/fcvm.2021.649094. PMID: 33928134; PMCID: PMC8076517.
- Dahiya DS, Wani F, Guidi JC, Kichloo A. Gastrointestinal Adverse Effects of Immunotherapeutic Agents: A Systematic Review. Gastroenterology Res. 2020 Dec;13(6):227-232. doi: 10.14740/gr1340. Epub 2020 Dec 23. PMID: 33447301; PMCID: PMC7781271.