Case Report
Near Complete Response to 177Lu-PSMA-DKFZ-617 Therapy in a Patient with Metastatic Castration Resistant Prostate Cancer

Madhav Prasad Yadav, Sanjana Ballal and Chandrasekhar Bal*
Department of Nuclear Medicine, All India Institute of Medical Sciences, New Delhi, India
*Address for Correspondence: Chandrasekhar Bal, Professor & Head, Department of Nuclear Medicine, AIIMS, Ansari Nagar, New Delhi -110029, India, Tel: +91-9868397182; Fax: 91-11-26588664; Email: csbal@hotmail.com
Dates: Submitted: 27 November 2017; Approved: 04 December 2017; Published: 05 December 2017
How to cite this article: Yadav MP, Ballal S, Bal C. Near Complete Response to 177Lu-PSMA-DKFZ-617 Therapy in a Patient with Metastatic Castration Resistant Prostate Cancer. J Radiol Oncol. 2017; 1: 083-086. DOI: 10.29328/journal.jro.1001012
Copyright License: 2017 Yadav MP, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Metastatic castration resistant prostate cancer; 68Ga- PSMA-HBED-CC PET/CT; 177Lu-PSMA-DKFZ-617 Radioligand therapy
Abstract
Prostate specific membrane antigen, a type II transmembrane protein is an excellent target for the radionuclide therapy in advanced prostate cancer patients due to its high expression in the prostate cancer cells. We present the case of a 69-year old man with advanced metastatic castration resistant prostate cancer. In view of rising serum PSA levels despite hormonal and chemotherapy, we decided to perform a 68Ga-PSMA-HBED-CC PET/CT scan (prostate specific membrane antigen). It revealed intense radiotracer uptake in the prostate, lymph nodes and multiple skeletal sites. Five cycles of 177Lu-PSMA-DKFZ-617 radioligand therapy were administered in the patient followed by an intrim 68Ga-PSMA-HBED-CC PET/CT. Intrim 68Ga-PSMA-HBED-CC PET/CT scan demonstrated a near complete remission of disease with a corresponding decrease in the sPSA levels. During the follow-up duration of 12 months, the patient did not develop haematological, kidney and liver toxicity during the course of treatment and follow-up. 177Lu-PSMA-DKFZ-617 is a promising therapeutic option in metastatic castration resistant prostate cancer (mCRPC) patients.
Introduction
Castration resistant prostate cancer is defined by disease progression despite androgen deprivation therapy and may present with rise in sPSA levels or progression of pre-existing disease with appearance of new lesions [1]. 10-20% of prostate cancer patients progress to mCRPC with 90% of them presenting with bone metastasis [2]. Docetaxel chemotherapy is considered as the standard of care in metastatic castration resistant prostate cancer (mCRPC) patients. Patients with disease progression regardless of docetaxel have limited therapy options. In patients who have exhausted all therapy options the introduction of targeted radionuclide therapy with 177Lu-PSMA-DKFZ-617 has opened a new horizon in the treatment of mCRPC. In this study, we present the course of treatment in a case of mCRPC patient treated 177Lu-PSMA-DKFZ-617 therapy.
Case Report
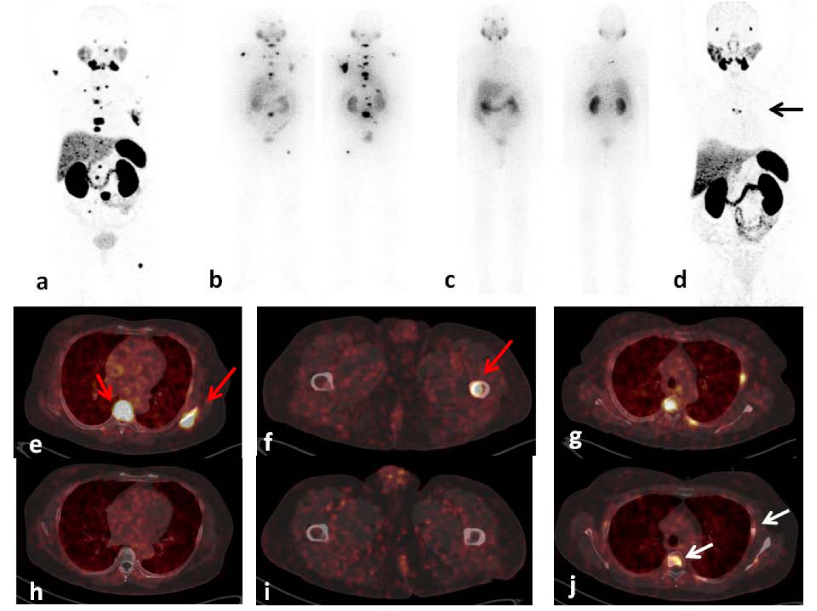
A 69-year old male was diagnosed as a case of carcinoma prostate in January 2007. Biopsy was suggestive of Adenocarcinoma (Gleason’s Grade 7). Magnetic resonance imaging showed enlarged prostate with hypointense lesion in the head of humerus. He was started on bicalutamide and injection Zoladex in 2007 and continued till 2012 which was succeeded by bilateral orchidectomy. During hormonal therapy, radiation therapy to the prostate by an intensity modulated radiotherapy technique was conducted. He received 45 Gy in 25 fractions to the whole pelvis followed by 30.6 Gy in 17 fractions to the prostate. His routine blood counts were found to be normal throughout the treatment. Despite hormonal therapy, patient presented with low back ache and rising sPSA levels (94 ng/ml). 99mTc-MDP whole body bone scan was performed with scan findings suggestive of multiple skeletal metastases involving the left scapula, B/L ribs, T4-5, T11,12 and L1-L5 vertebrae. 40 fractions of radiotherapy from cervical to lumbar spine were delivered followed by Honvan 120 mg thrice a day till October 2014. Subsequently, 4 cycles of Docetaxel chemotherapy was administered but discontinued and switched to Abiraterone treatment due to persistently rising sPSA levels (156 ng/ml) and continued till November 2016. The patient was referred to the department of Nuclear Medicine for further management. Baseline 68Ga-PSMA-HBED-CC PET/CT was performed for the staging of disease and scan features were suggestive of heterogeneous tracer uptake in the prostate, with sub-centimetric bilateral common iliac, paraaortic lymph nodes with mild tracer uptake and avid tracer uptake in multiple sclerotic skeletal metastases (Figure 1). Based on 68Ga-PSMA-HBED-CC PET/CT findings, patient was scheduled for 177Lu-PSMA-617 radioligand therapy. Interestingly, biochemical partial response was noted immediately after 1st cycle of 177Lu-PSMA-DKFZ-617 therapy (3700 MBq) with reduction of sPSA levels from 246 ng/ml to 108 ng/ml (Figure 1b). subsequently, four cycles of 177Lu-PSMA-DKFZ-617 radioligand therapy (cumulative activity: 14800 MBq) were administered to the patient followed by an intrim 68Ga-PSMA-HBED-CC PET/CT. Intrim 68Ga-PSMA-HBED-CC PET/CT scan demonstrated a near complete remission(Figures 1d,1h,1i) of disease with further decreases in the sPSA levels (108 ng/ml to 2.5 ng/ml, P<0.000). The patient did not develop haematological, kidney and liver toxicity during the course of treatment and follow-up. The VASmax score, KPS, AS and laboratory parameters were evaluated in the patient before therapy, and at follow-up ranging from 2 weeks to 3 months after every cycle of 177Lu-PSMA-DKFZ-617 therapy. The visual analgesic maximum score (VASmax) improved from 9 to 1. An improvement in the Eastern Cooperative Oncology Group (ECOG) status (3 to 1) and Karnofsky Performance Score (KPS) (40 to 80) score was also noted. The patient is on regular follow-up and has shown an extended survival with improved quality of life.
Figure 1: A 69-year-old prostate cancer patient treated hormonal and chemotherapy presented with radiotracer avid extensive skeletal and lymph node metastasis on pre-therapy diagnostic 68Ga-PSMA-HBED-CC PET/CT scan [a,e,f,g, (red arrows)] and 1st cycle of 177Lu-PSMA-DKFZ-617 24 hr anterior and posterior whole body scintigraphy (WBS) (b). After the 5th cycle the post-therapy 177Lu-PSMA-DKFZ-617 scan revealed complete resolution of all lesions except with residual disease in D4 vertebra and left scapula (c). Intrim 68Ga-PSMA-HBED-CC PET/CT scan demonstrated decreased PSMA uptake, size and number of lesions (d,h,i) with residual disease in the D4 vertebra, left 5th rib and left scapula [d (black arrow), j(white arrows)] consistent with near complete response.
During the duration of 1 year, patient follow-up was performed at 2 weeks, 1 month and 3 months after each cycle of 177Lu-PSMA-DKFZ-617 therapy with hematological, kidney function, glomerular filtration rate (GFR) and liver function to assess the toxicity according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3 [3]. The patient was on regular quarterly follow-ups with monitoring of serum PSA levels. No haematological, kidney and liver toxicity was observed in the patient.
Discussion
Prostate specific membrane antigen (PSMA) is a membrane bound protein that is more avidly expressed in primary as well as in metastatic prostate cancer [4-6]. Extensive research is going on to develop PSMA ligands. However, all the PSMA ligands used share similar Glu-urea motif which binds to the proteolytic domain and a lipophilic chelate. Unlike PSMA-HBED-CC ligand used for the imaging of prostate cancer, ligands such as PSMA-DKFZ-617 and PSMA I&T [7] have been developed for targeted radioligand therapy with similar therapeutic efficacy.
The introduction of 68Ga-PSMA-HBED-CC PET/CT imaging has remarkably improved the accuracy of restaging the disease in recurrent prostate cancer [8]. Currently, 68Ga-PSMA-HBED-CC PET/CT is the most widely accepted agent in the imaging of recurrent prostate cancer. In this patient, we observed an excellent correlation between intrim 68Ga-PSMA-HBED-CC PET/CT scans and sPSA response. In agreement with our findings, a retrospective analysis involving 1007 recurrent prostate cancer patients demonstrated a strong association of tumour detection and radiotracer avidity with sPSA levels [8].
Few preclinical studies have demonstrated 177Lu-PSMA-DKFZ-617 as a promising agent for targeted internal radiation therapy [9]. 177Lu-PSMA-DKFZ-617 therapy has rapidly gained global importance during last five years due to its proven effectiveness in the prolonged overall survival and safety with no major toxicities [7,10-15]. It is a widely established therapy option in the entire Europe and Asia, while in USA, the first multi-center phase II clinical trial of 177Lu-PSMA-DKFZ-617 RLT in mCRPC has recently received its FDA clearance.
A series of dosimetry studies have documented 177Lu-PSMA-DKFZ-617 RLT safe with minimal absorbed dose to kidney [14-16]. Subsequently several clinical studies including our recently published articles have demonstrated excellent response to 177Lu-PSMA-DKFZ-617 RLT [7,10-15]. The patient in the current report has been followed up in our department since 1 year and attained normalization of sPSA levels with corresponding near complete resolution of disease. The long tumor retention and low kidney uptake makes 177Lu-PSMA-DKFZ-617 therapy as an excellent choice for therapeutic application in hormone refractory metastatic prostate cancer patients.
Similar to our results, studies have confirmed a notable improvement in the quality of life from 177Lu-PSMA-DKFZ-617 RLT [7,10,12]. Additionally, in concordance studies have proved 177Lu-PSMA-DKFZ-617 RLT safe with negligible toxicities and side effects in hormone refractory metastatic prostate cancer patients [12].
In our institution 177Lu-PSMA-DKFZ-617 therapy is carried out on a forth nightly basis. Since 2014, we have treated 68 mCRPC patients and have observed a partial response rate of 52%. Including the current patient, 3 patients have achieved near complete response. This indicates that 177Lu-PSMA-617 therapy is promising systemic therapy option in patients with metastatic castration resistant prostate patients with limited therapy options. 177Lu-PSMA-DKFZ-617 is feasible, effective, safe and presumed to be cost-effective therapeutic option in CRPC patients where access to alpha therapy is limited.
References
- Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010; 17: 72-79. Ref.: https://goo.gl/fnZuRe
- Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systemic review. Int J Clin Pract. 2011; 65: 1180-1192. Ref.: https://goo.gl/UJ2PXY
- Green S, Weiss GR. Southwest Onology group standard response criteria, endpoint definitions and toxicity criteria. Invest new Drugs. 1992; 10: 239-253. Ref.: https://goo.gl/EfnvAK
- Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of PCa: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013; 40: 486-495. Ref.: https://goo.gl/ZNDQFU
- Minner S, Wittmer C, Graefen M, Salomon G, Steuber T, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. 2011; 71: 281-288. Ref.: https://goo.gl/4m3X6d
- Rybalov M, Ananias HJ, Hoving HD, van der Poel HG, Rosati S, et al. PSMA, EpCAM, VEGF and GRPR as imaging targets in locally recurrent prostate cancer after radiotherapy. Int J Mol Sci. 2014; 15: 6046-6061. Ref.: https://goo.gl/QNrNbm
- Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, et al. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J Nucl Med. 2016; 57: 1006-1013. Ref.: https://goo.gl/vBz8DZ
- Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017; 44: 1258-1268. Ref.: https://goo.gl/HmbeA6
- Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J Nucl Med. 2015; 56: 1697-1705. Ref.: https://goo.gl/mwdodQ
- Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, et al. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J Nucl Med. 2016; 57: 1170-1176. Ref.: https://goo.gl/Ehvsmj
- Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med. 2017; 58: 85-90. Ref.: https://goo.gl/zptRHE
- Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, et al. (177)Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017; 44: 81-91. Ref.: https://goo.gl/z3GMyq
- Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2017; 45: 12-19. Ref.: https://goo.gl/gKxd59
- Kabasakal L, Toklu T, Yeyin N, Demirci E, Abuqbeitah M, et al. Lu-177-PSMA-617 Prostate-Specific Membrane Antigen Inhibitor Therapy in Patients with Castration-Resistant Prostate Cancer: Stability, Bio-distribution and Dosimetry. Mol Imaging Radionucl Ther. 2017; 26: 62-68. Ref.: https://goo.gl/vyDNeE
- Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, et al. Dosimetry for (177)Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016; 43: 42-51. Ref.: https://goo.gl/Kdwi38
- Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, et al. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl Med Commun. 2017; 38: 91-98. Ref.: https://goo.gl/DbHCt6